Quality



Continuous Improvement of the Quality Management System Throughout the Entire Clinical Trial Lifecycle
Meeting and Exceeding Quality Goals for Pharmaceutical Innovation
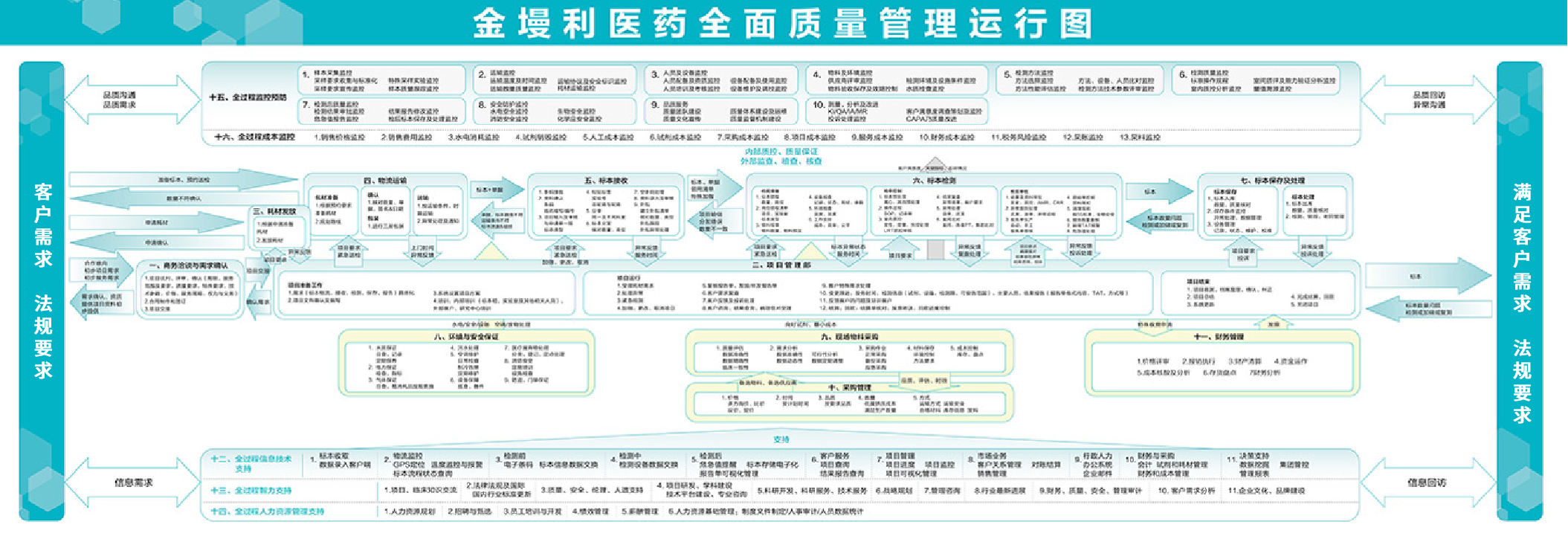
KingMylab strictly adheres to relevant laws, regulations, international quality standards, and industry norms, including GCP, GCLP, CAP, ISO15189, and ISO9001. In doing so, the company establishes a comprehensive quality management system that encompasses the entire chain and all processes of its clinical trial center laboratory services. Guided by the quality policy of "Fairness and Compliance, Authenticity and Rigor, Scientific Accuracy," KingMylab promotes a quality culture of "Integrity, Truth-Seeking, Continuous Improvement" within the organization. The company has established an advanced quality organization with dedicated and professional quality personnel, covering quality functions from quality strategy to quality assurance, quality auditing, and quality control. A comprehensive quality management system is in place, including document management, process management, quality metric measurement, quality auditing, continuous quality improvement, and major quality issue escalation systems. KingMylab maintains a database of nearly 200 QA/QC-related documents, over 120 business processes, nearly 90 quality metrics, and conducts over 100 internal and external audits annually. We are committed to building a preventative quality management system throughout the entire clinical project lifecycle, meeting and exceeding quality goals for pharmaceutical innovation, and achieving an industry-leading quality operations system.