Central Lab
Central laboratory testing is ideal for pharmaceutical trials, especially multi-center studies, as most on-site laboratories lack the means to perform sensitive, validated, and regulations-compliant testing regarding drug safety and efficacy. In addition, protocol, reference range, and analysis differences among local labs may result in unacceptable levels of bias and variation in the trial data, obstructing meaningful interpretation.
As an experienced industry leader, KingMylab has established an internationally recognized clinical trial testing and management system, including CAP, ISO15189, GCP and GLP compliance under the 21CFR Part 11 regulation, to ensure the authenticity, integrity and accuracy of experimental data. With more than 82 technical platforms, 4,000+ validated tests, and more in development, KingMylab offers one-stop central laboratory and bioassay solutions to satisfy all your clinical trial testing needs from Phase I to Phase IV.
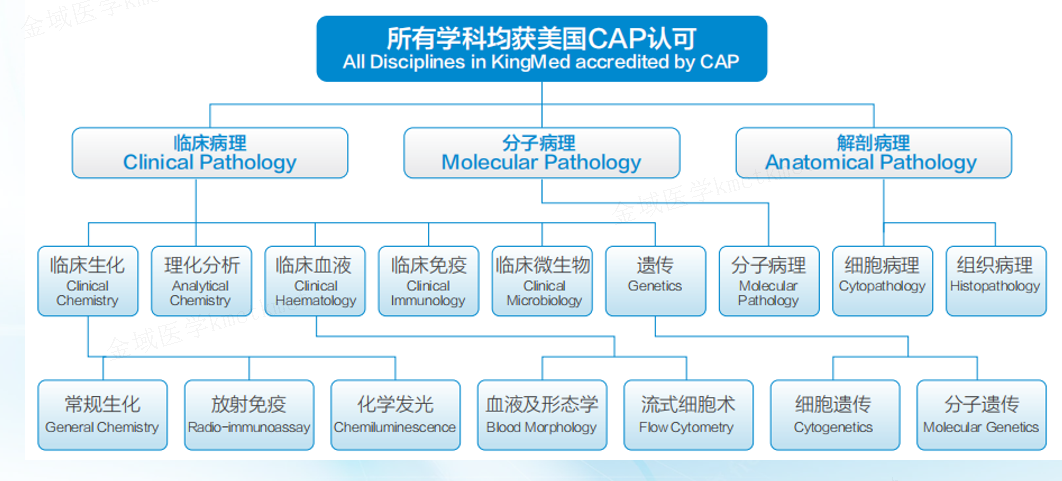

Pathology and Immunohistochemistry Center has always been in line with international standards, the center has established a perfect quality management system in accordance with the requirements of CAP and ISO15189, and established characteristic pathological subspecialties (such as cell pathology, kidney pathology, skin pathology, lymphatic hematopoietic pathology, liver pathology, head and neck pathology, digestive pathology, gynecological pathology, thoracic and lung pathology, etc.). Each subspecialty performs pathological diagnosis and sends pathological reports according to the requirements of domestic and foreign guidelines, combined with the detection of characteristic technology platforms (such as immunohistochemistry, electron microscopy, fluorescence, flow cytometry and molecular pathology detection, etc.). At present, the center has undertaken more than 650 clinical trial projects, including routine pathological examination and diagnosis, cytology examination and diagnosis, immunohistochemical staining and diagnosis, special staining and diagnosis, and remote pathological diagnosis.

The center has a group of more than 100 pathologists, including more than 10 overseas pathologists. In addition to pathological diagnosis doctors, the center also has more than 30 pathological technicians. The center attaches great importance to the introduction and training of pathological talents, and the training courses cover pathological techniques, gross specimen examination, microscopic examination, immunohistochemistry, molecular pathology and pathological diagnosis.


1. Remote Pathology Film Reading System
We have a self-developed remote pathology film reading system, which can customize the system functions according to the requirements of the research scheme, to ensure data security and the independence of Independent Review Committee (IRC) film reading. The pathologists participating in IRC are all senior doctors in the pathology subspecialty of the disease field. The project is carried out in strict accordance with SOP, and the system meets the requirements of FDA/NMPA and 21 CFR part 11.
So far, our Pathology IRC has successfully participated in the research and development of more than 10 drugs, and the number of pathology projects at the center has exceeded 650, which has rich project experience in lung cancer, breast cancer, stomach cancer, bladder cancer, cervical cancer and other disease fields, and can organize the corresponding IRC assessment according to the pathological criteria required in the design of clinical trial protocols, and conduct quality control of pathological diagnosis results. Our goal is to assist new drug research and development, drug effect evaluation and prediction through digital pathology, to ensure accurate and reliable results, so as to improve the efficiency and quality of drug research and development, and accelerate the pace of drug marketing.
2. Remote Digital Section Scanning Platform
The platform integrates digital section scanning, microscopic image processing, Web image browsing and other technologies, combined with senior expert resources in the field of pathology. It can provide convenient, time-saving, labor-saving and rapid pathological diagnosis services, effectively breaking through the limitations of time and space. At the same time, it supports multiple experts to carry out remote consultation, discuss and diagnose complex and difficult cases, and further improve the accuracy of diagnosis. It also supports the export of pathological diagnostic data for in-depth analysis and statistics.
3. AI+ Computational Pathology
In order to improve the accuracy of pathological interpretation and reduce the difference between pathologists' reading of pictures, the center applied the first intelligent microscope product approved by the National Food and Drug Administration (NMPA) in China, which was developed in cooperation with Tencent AI Lab and Sainyu Optical Technology. AI+ computational pathology centered on pathologists, research and exploration in AI have assisted protein expression analysis and other aspects.
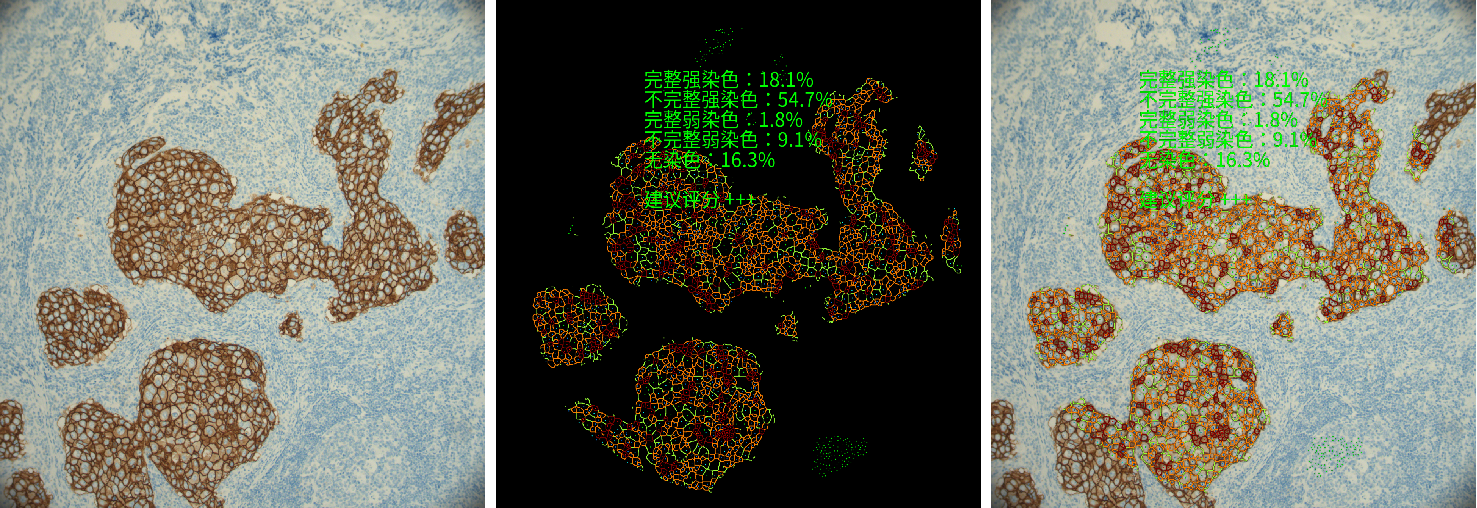
eg:HER2 Precise quantitative analysis
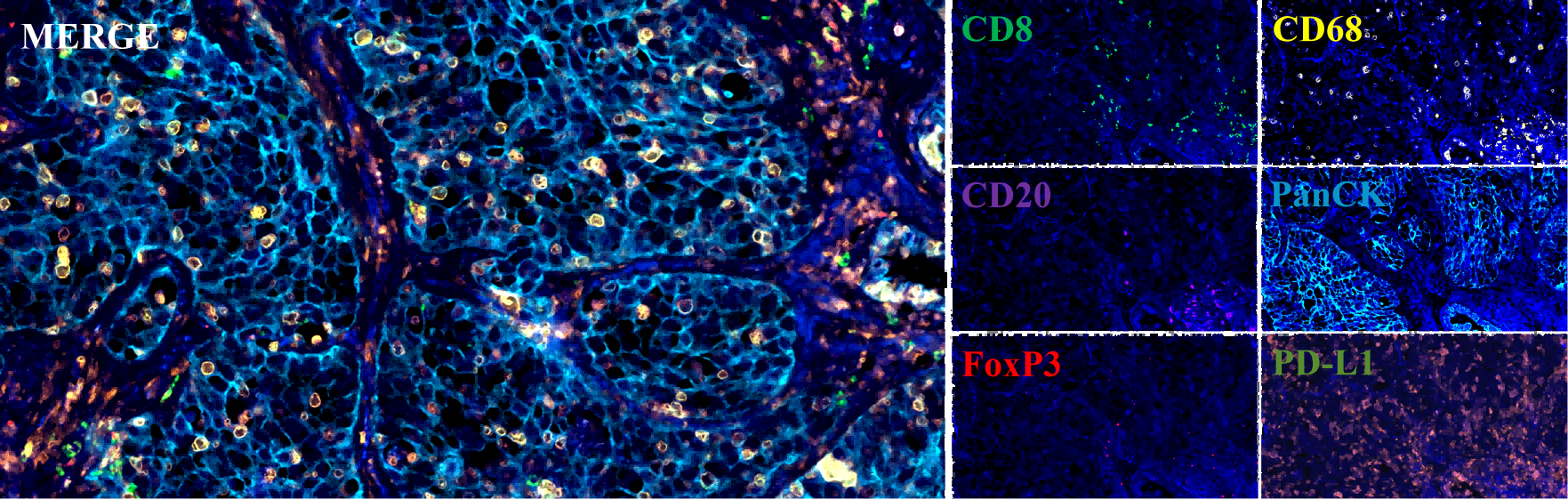
4. Multiplex Immunohistochemical Platform (mIHC)
Based on the tyramide signal amplification technology (TSA), the mIHC platform develops a set of multi-label redyeing scheme. The self-developed mIHC Panel follows the system and standard requirements such as GLP, CAP, and CIMAC standards, including 11 verified mature panels. It involves more than 35 popular targets and supports more labeling (8-10 labels) and spontaneous fluorescence recognition. At the same time, the platform provides strong support for the detection of tumor microenvironment biomarkers or adjuvant chemotherapy and immune checkpoint therapy.
5. Immunohistochemical Platform (IHC)
The center is equipped with automatic immunohistochemical staining machines such as Roche, Agilent and Leica, and has completed the development and verification of hundreds of markers, and can support the development needs of more new markers.