Central Lab
Central laboratory testing is ideal for pharmaceutical trials, especially multi-center studies, as most on-site laboratories lack the means to perform sensitive, validated, and regulations-compliant testing regarding drug safety and efficacy. In addition, protocol, reference range, and analysis differences among local labs may result in unacceptable levels of bias and variation in the trial data, obstructing meaningful interpretation.
As an experienced industry leader, KingMylab has established an internationally recognized clinical trial testing and management system, including CAP, ISO15189, GCP and GLP compliance under the 21CFR Part 11 regulation, to ensure the authenticity, integrity and accuracy of experimental data. With more than 82 technical platforms, 4,000+ validated tests, and more in development, KingMylab offers one-stop central laboratory and bioassay solutions to satisfy all your clinical trial testing needs from Phase I to Phase IV.
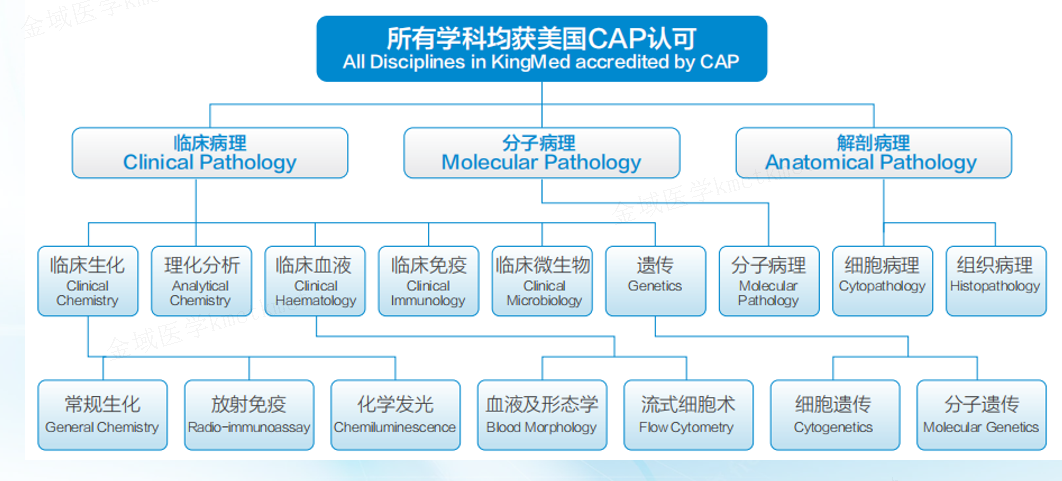

The Gene and Genome Center integrates Cytogenetics, Molecular Genetics, Molecular Pathology, Molecular Infectious Disease and Bioinformatics into a central, comprehensive testing platform for clinical studies. The center is in accordance with the requirements of the CAP and ISO15189. As one of the largest and most advanced clinical genetics laboratories in China, the facility offers well over 1000 tests and has supported more than 500 clinical projects in fields as diverse as oncology, heriditary and rare diseases, infection, and chronic conditions. It provides testing services for the diagnosis, prediction, and prognosis of genetically-related illnesses and the monitoring of drug safety and efficacy in clinical trials. In addition, the center can aid in the development of novel biomarkers.

To provide one-stop support for your genetic and genomic testing, our team is as diverse as our services. Our technical staff includes laboratory technicians, bioinformaticians, clinical specialists, and data engineers among others. The leadership comprises of technical and medical experts with extensive research and clinical experiences, both in China and overseas, in the diagnosis and treatment of human genetic diseases. In addition, all team members are well-trained in clinical laboratory management and quality control and participate regularly in career development sessions.

1. NGS
The center is equipped with a range of Illumina and Thermo Fisher NGS instruments, including the NovaSeq 6000 and Ion S5, as well as one third-generation PacBio sequencer. Combining the core analysis algorithms developed and verified by the bioinformatics team, a multi-omics biomarker discovery pipeline, and a knowledge database built by the clinical team, the lab offers targeted enrichment sequencing, whole exome sequencing (WES), whole genome sequencing (WGS), whole transcriptome sequencing among others.

2. PCR
Various real-time fluorescent quantitative PCR instruments such as the Rotor-Gene Q, ABI 7500, Roche z480, Bio-Rad™QX200™ PCR and Roche Digital LightCycler System are available and have been used in genotyping, copy number analysis, and analysis of microorganisms. Digital PCR technology is also available and has been applied to detection of low abundance mutations and absolute quantitation of pathogens.
3. Karyotype analysis
The center provides several sets of Carl Zeiss automatic chromosome scanners and analysis software, operated by technicians with at least 5 years of experience, for karyotype analysis.

4. Fluorescence in Situ Hybridization Technology (FISH)
Fluorescence in situ hybridization (FISH) is used to visually detect the number and structural variation of known genes or chromosomes in human cells or tissues using fluorescently labeled probes. The center offers nearly 100 FISH tests.
5. Sanger sequencing
Sanger sequencing is the classic direct sequencing method, and is still the "gold standard" of sequencing. In the genetic diagnosis of hereditary diseases, Sanger sequencing is often used to confirm the results of NGS.