Central Lab
Central laboratory testing is ideal for pharmaceutical trials, especially multi-center studies, as most on-site laboratories lack the means to perform sensitive, validated, and regulations-compliant testing regarding drug safety and efficacy. In addition, protocol, reference range, and analysis differences among local labs may result in unacceptable levels of bias and variation in the trial data, obstructing meaningful interpretation.
As an experienced industry leader, KingMylab has established an internationally recognized clinical trial testing and management system, including CAP, ISO15189, GCP and GLP compliance under the 21CFR Part 11 regulation, to ensure the authenticity, integrity and accuracy of experimental data. With more than 82 technical platforms, 4,000+ validated tests, and more in development, KingMylab offers one-stop central laboratory and bioassay solutions to satisfy all your clinical trial testing needs from Phase I to Phase IV.
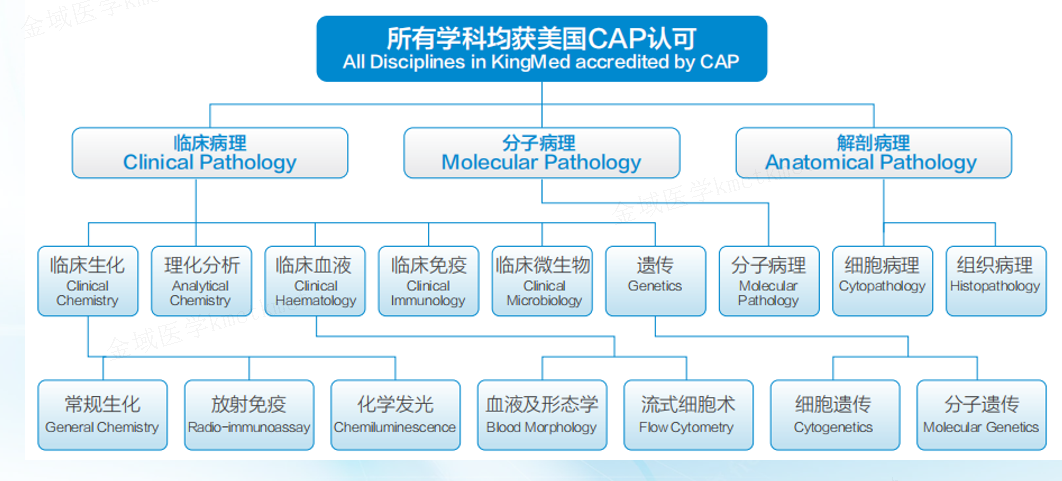

Pathogen microorganism culture and drug sensitive combination is a comprehensive testing laboratory composed of morphology, culture, immunoserology, molecular biology and other technical platforms. It mainly detects samples from various parts of the human body, including bacteria, fungi, parasites and viruses. The platform has established a sound quality management system in accordance with the requirements of CAP and ISO15189, and has become a large-scale medical microbial detection service platform with multi-platform integration, complete detection items and a wide range of pathogens. At present, more than 800,000 samples are tested annually, and more than 160 testing items are carried out.

At present, there are more than 20 testing personnel, distributed in sample pretreatment, bacteria, fungi and divergences bacteria detection, morphological examination, infection immunity and other testing positions, of which more than 90% of the personnel have obtained the inspection title certificate.

The platform has a variety of culture systems such as ordinary constant temperature, anaerobic micro-aerobic, automatic blood culture, etc., which can meet the culture of viruses, mycobacteria, bacteria, fungi and other microorganisms. The species were identified by morphological identification, MALDI-TOF mass spectrometry identification, serological detection and Sanger sequencing. The application of automatic bacterial identification and drug sensitivity analysis system, as well as mature fungal and viral drug sensitivity detection system, can realize the drug sensitivity detection of common drugs and the new ones.