Central Lab
Central laboratory testing is ideal for pharmaceutical trials, especially multi-center studies, as most on-site laboratories lack the means to perform sensitive, validated, and regulations-compliant testing regarding drug safety and efficacy. In addition, protocol, reference range, and analysis differences among local labs may result in unacceptable levels of bias and variation in the trial data, obstructing meaningful interpretation.
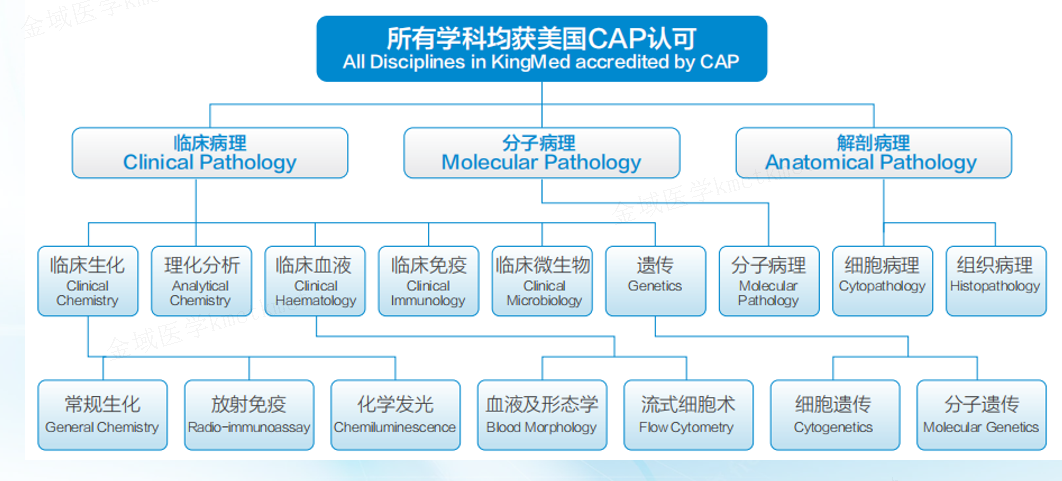
As an experienced industry leader, KingMylab has established an internationally recognized clinical trial testing and management system, including CAP, ISO15189, GCP and GLP compliance under the 21CFR Part 11 regulation, to ensure the authenticity, integrity and accuracy of experimental data. With more than 82 technical platforms, 4,000+ validated tests, and more in development, KingMylab offers one-stop central laboratory and bioassay solutions to satisfy all your clinical trial testing needs from Phase I to Phase IV.

Our industry leading flow cytometry core is among the first clinical FACS centers in China as well as the first to obtain CAP and ISO15189 certification. To date, the flow cytometry lab has developed and validated more than 150 testing panels, ranging from immune cell phenotyping to minimal residual disease assessment in hematological malignancies. The center has supported more than 300 clinical trial projects in areas such as hematopoietic and lymphoid tumors, non-tumorous hematological diseases, and immunotherapy. In addition, it offers custom panel design and validation services to suit the individualized needs of your clinical project.

With nationally and internationally renowned clinical cytometry experts leading a core team of experienced technicians and analysts, the FACS center aims to deliver the highest level of expertise and support to your project. We are keenly aware of the importance of skilled personnel in ensuring the quality of complex cytometric experiments and thus we are deeply committed to talent development, providing a rigorous training course and lifetime learning opportunities to all of our staff. In fact, through our program, we trained China's first generation of hematopathologists (GPS physicians), by integrating the central platforms of Morphology, Immunology, Cytogenetics and Molecular Biology, and introducing China's first GPS (Genetic Pathology Solution) multi-platform diagnostic service.

Currently, the center is equipped with 14 multi-parameter, high-throughput clinical flow cytometers and 1 38-channel full spectrum cytometer from Becton Dickinson and Beckman Coulter.