Central Lab
Central laboratory testing is ideal for pharmaceutical trials, especially multi-center studies, as most on-site laboratories lack the means to perform sensitive, validated, and regulations-compliant testing regarding drug safety and efficacy. In addition, protocol, reference range, and analysis differences among local labs may result in unacceptable levels of bias and variation in the trial data, obstructing meaningful interpretation.
As an experienced industry leader, KingMylab has established an internationally recognized clinical trial testing and management system, including CAP, ISO15189, GCP and GLP compliance under the 21CFR Part 11 regulation, to ensure the authenticity, integrity and accuracy of experimental data. With more than 82 technical platforms, 4,000+ validated tests, and more in development, KingMylab offers one-stop central laboratory and bioassay solutions to satisfy all your clinical trial testing needs from Phase I to Phase IV.
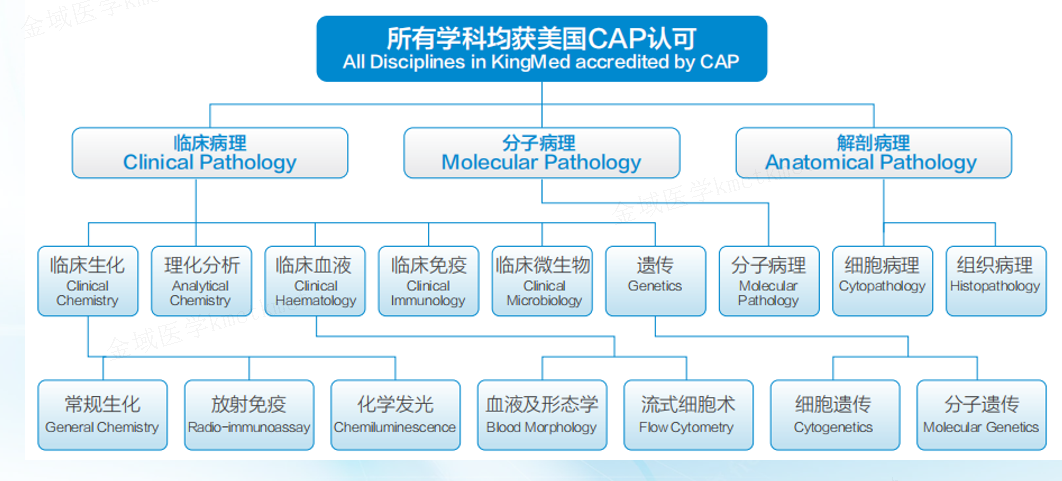

The biochemical immune testing center consists of clinical biochemical laboratory, chemiluminescence laboratory and immune laboratory. The Center strictly manages the laboratory in accordance with ISO15189 and the quality management system requirements of the CAP. At present, more than hundreds of testing items such as blood glucose, blood lipids, liver and kidney function, myocardial enzyme profile, electrolyte, rheumatism immunity, and special protein have been carried out, and the number of projects is in the leading level in the country. The center has undertaken more than 1,000 clinical trial projects, supported more than 100,000 clinical trial samples, and provided testing indicators such as safety, pharmacodynamics and efficacy for clinical trials. In addition to the validated tests, the Center can support the development of new markers

The discipline leaders and laboratory technical leaders of the center have more than 15 years of clinical laboratory work experience, and the technical backbone of the center has an average of 5 years of clinical laboratory work experience, which escorts the test quality of the clinical trial.

The center is equipped with a number of automatic biochemical instrument, automatic chemiluminescence instrument, automatic enzyme immunity instrument, HELIOS automatic indirect fluorescence immunoanalyzer, Phadia 1000 automatic allergen analyzer, automatic immunoblotter and other large automatic testing equipment, as well as various enzyme label instrument, fluorescence microscope, etc. Equipped with a large Roche automatic biochemical immune line, the detection speed reaches 10,000 test/hour, which greatly improves the detection efficiency and quality of the laboratory.